Design Process
Developing a new products is a complex and risky journey that requires good planning and execution. A product development journey is uncertain and entails risks that jeopardize the outcome. Resources are always limited, time is critical and quality needs to be exceptional. A good design process with clear milestones is the best tool that can be used to achieve outstanding results. The design process that I follow seeks continuous review of risks as the design materializes setting up defined deliverables in terms of design definition, technology readiness and consumer feedback at each point in the process.
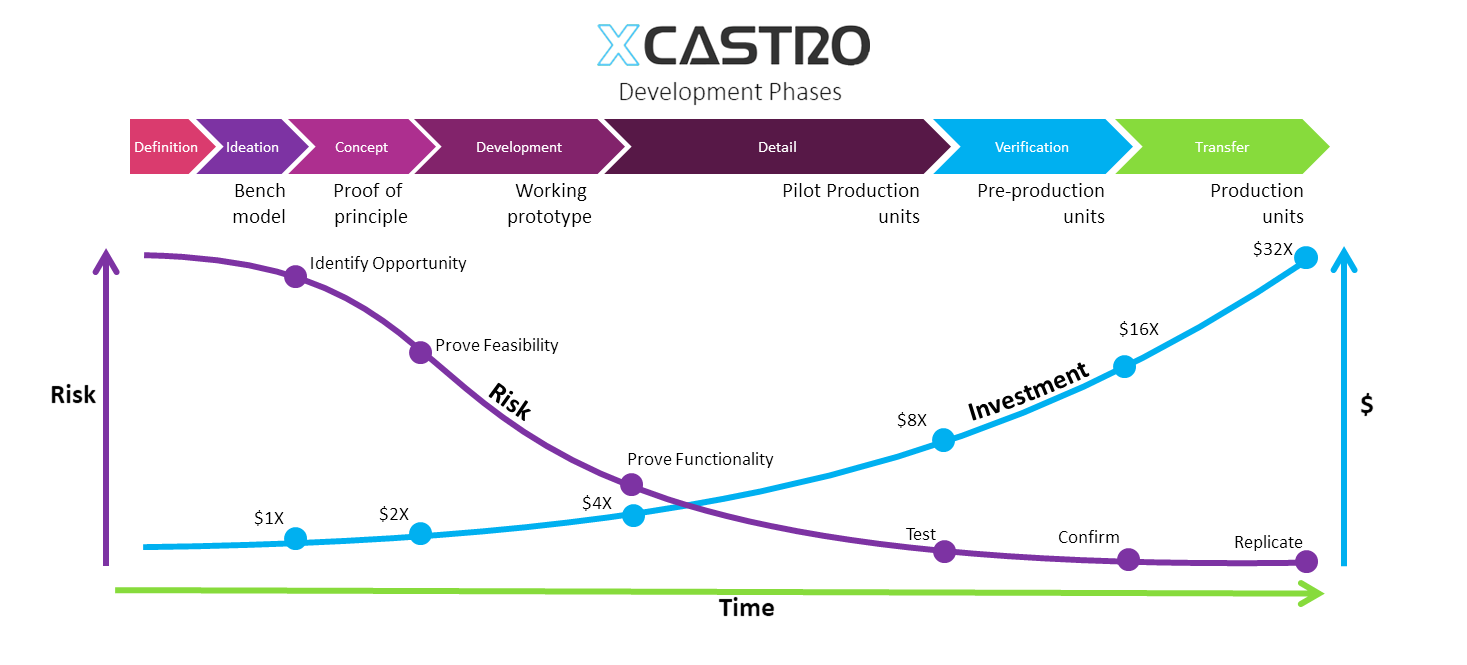
Stage –Gate phases
The process that I follow aligns with most companies’ phases of work and follows the traditional TRL (technology readiness levels). This process is divided in 7 phases or stages that have discreet outcomes. Each outcome looks on de-risking particular technological and design aspects of the product. Some of which are achieved by technical demonstrators or prototypes and other by user trials.
Risk Management
In an ideal world we would have unlimited resources when developing a product and we could work in all the aspects of a product at the same time to finish the project quickly. However this is impossible, resources are always limited and if we were to work on all aspects at the same time, we would only go round and round in circles increasing the complexity without reaching any solution. This situation forces us to “pick battles”, one at a time, with the aim of fighting the hardest ones at the beginning of the journey and leaving the easy ones at the end. The reason behind this, is that if we are to fail, we want to fail quickly and with low financial exposure. Giving us also the opportunity to change quickly and adapt to uncertainties on early phases and defining the details as the product evolves.
Change is inevitable in a design journey and the cost of change increases exponentially as the product is defined. The idea behind the process that I follow is to make the most critical changes in early phases and have very limited number of changes in the last phases. Change will come from three main aspects; technological (this means to achieve the function), user (to meet the consumer needs) and manufacturing (to be able to make it at a competitive cost). Wirth my design process I continuously look into all this aspects to mitigate risks on all this areas to increase the potential of success.
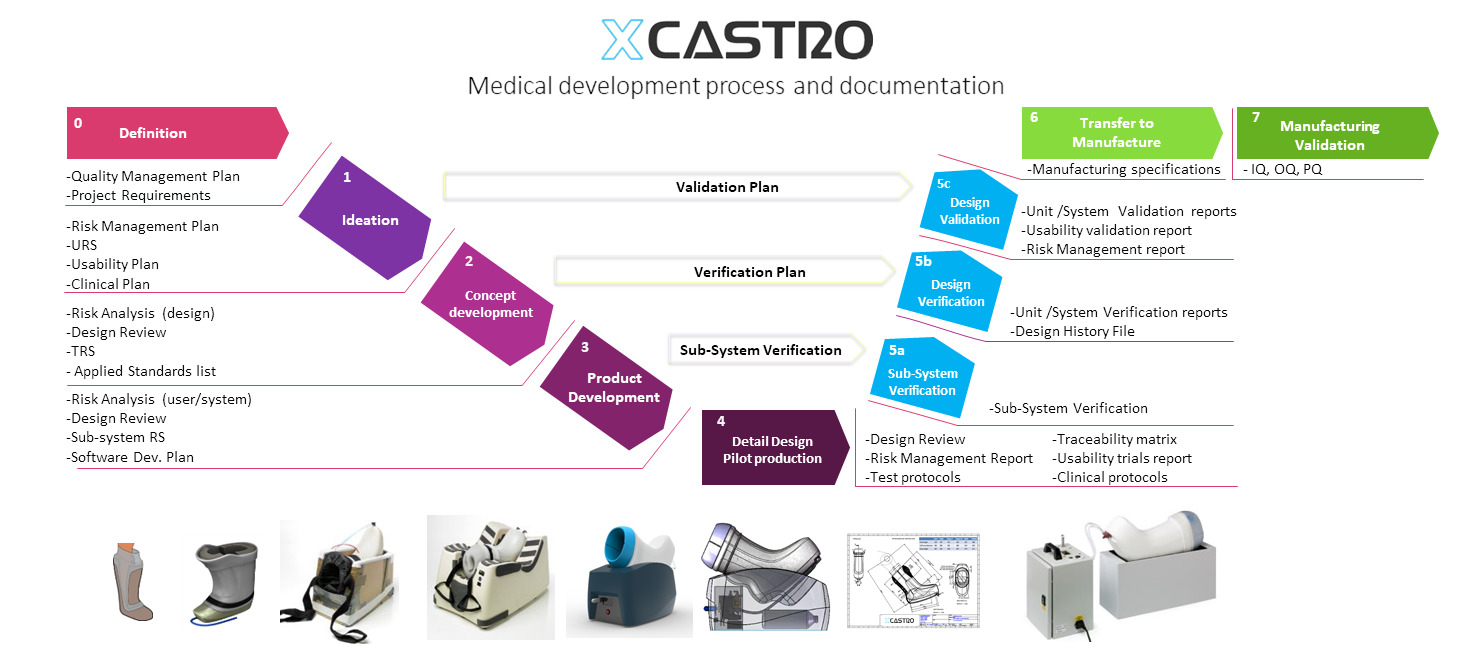
Medical development
Developing medical products requires a higher level of control and to define that not only technical, user and manufacturing aspects are being addressed but to have the required documentation that proves it, showing that all decisions have been made with the patient’s safety in mind.
Medical products vary on their documentation requirements but as rule of thumb I produce certain documentation at particular phases of work. This allows me to manage work load and reduce the risk of redesign or produce additional documentation or testing for the lack of early planning. The process that I follow aligns with ISO 13485, CE marking and FDA requirements. It resembles the V model, but also follows the cascade process.
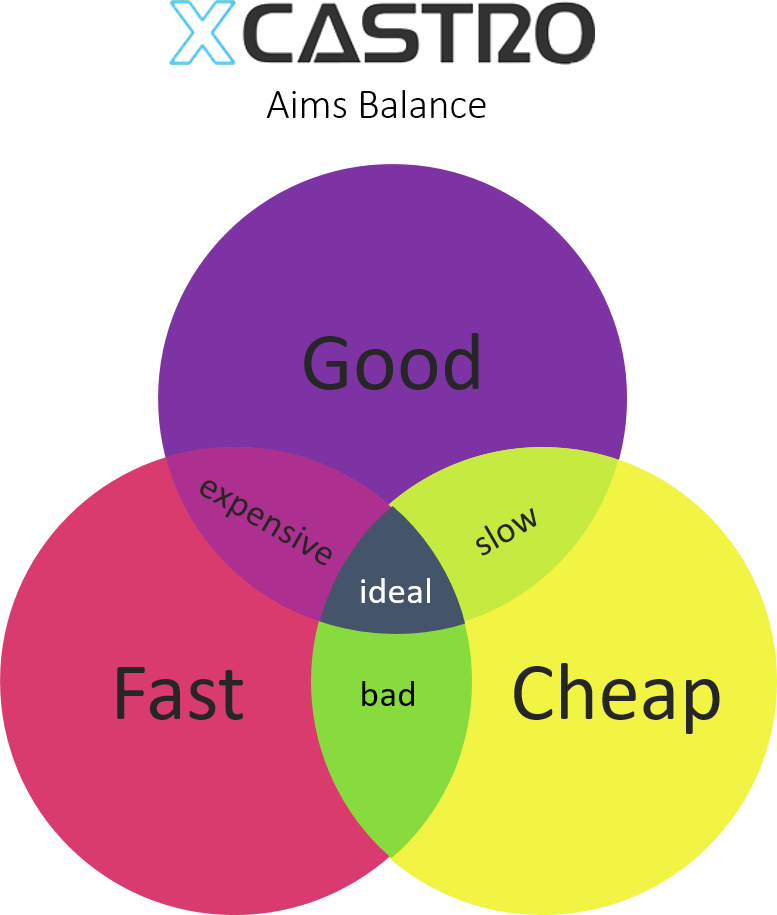
The ideal balance
In an ideal world, we would like design projects to be cheap, fast and of exceptional quality, but the reality is that often a compromise has to be done in one of this areas. As much as possible I try to balance and provide a good level on each of this aspects. Depending on each project requirements timescales can be extended or resources can be increased to meet stakeholder’s expectations. However, quality has to always be at the top of the priorities and never compromised.