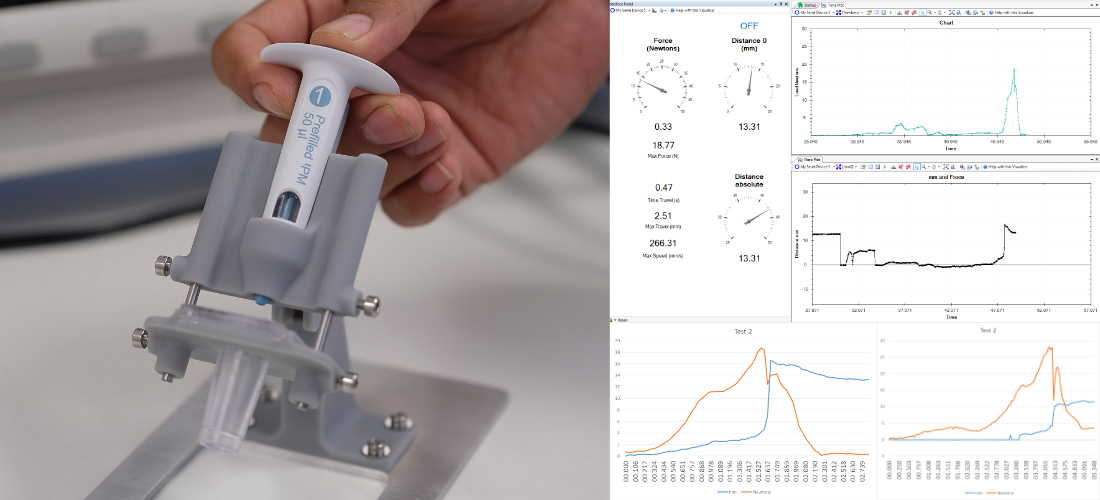
Testing and verification
Product testing to ensure performance, reliability, meet requirements and fulfil regulations.
Engineering test
Testing and validating our design and product is as important as designing it. The first point is to have well defined requirements that will outline how much testing will be required and the thoroughness demanded.
For some consumer and industrial products, a simple functional test will satisfy, however in other cases like medical products and high-volume consumer electronics thorough and methodical testing is required to identify early on potential design flaws.
My experience in testing, allows me to identify the required tests that will be cost effective to verify the product performance and ensure the best quality.
.jpg)
Test and verification Plan
Ideally, we want to define the tests as we design the product and test as early as we can, as changes are more expensive and complicated as the product evolves. A clearly defined test plan helps us to identify the resources and samples that will be needed to conduct tests and if needed book test external services. Also we will be able to develop the test jigs and equipment that will be needed and validate it.
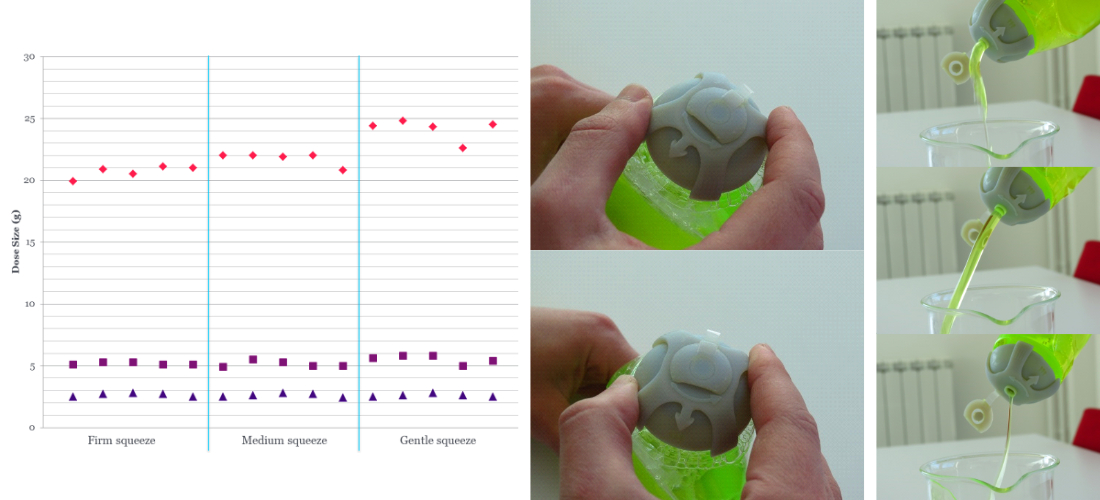
Test validation
Using methods such as Gauge R&R we will ensure that the test meets the requirements and will be reliable, repeatable, and reproducible.
Design Verification
This is perhaps the most critical aspect of a medical device development programme as it will demonstrate that the product is fit for purpose and safe to use. Testing has to be conducted following a series of standards, with validated protocols using gage R&R or other methodologies and calibrated equipment. Planning in this area is essential and having a good verification strategy and plan will reduce costs and timescales considerably. My experience in this area covers from drug delivery devices to class III caseworks.