Manufacturing Engineering and Industrialization
Experienced manufacturing consultant in London with 20+ years of experience in product development and NPI. Excellent understanding of injection moulding, casting, metal work, silicone and rubber moulding and prototyping. Used to work close to contract manufacturers in improving processes.

Manufacturing is perhaps the most risky aspect of product development. Not because things go wrong, but because usually challenges are underestimated, and the unplanned work and costs end ups terminating a project.
In my experience everything is possible to make, as long as there are enough resources (money and time) to develop a process technology and the law of physics are not defied. New materials and processes keep emerging, that prove this, but are often too expensive and time consuming to be adopted by non- specialized products.
My approach in this field is to first stick with the known. Use conventional materials and processes as much as possible and only when this is not possible, then look into adapting an existing process. Ultimately if all fails, developing your own processes.
My experience across several sectors and industries allows me to recommend the most convenient material and process from an early stage, reducing with this developing time and costs. Also, my experience allows me to help clients and companies on stablishing the required specifications, approval processes and quality systems to ensure that a new product is made the right way from the beginning.
In addition, my network of international suppliers allows me to recommend or trusted manufacturers that can be a good match for each project.
Design for manufacturing (DFM)
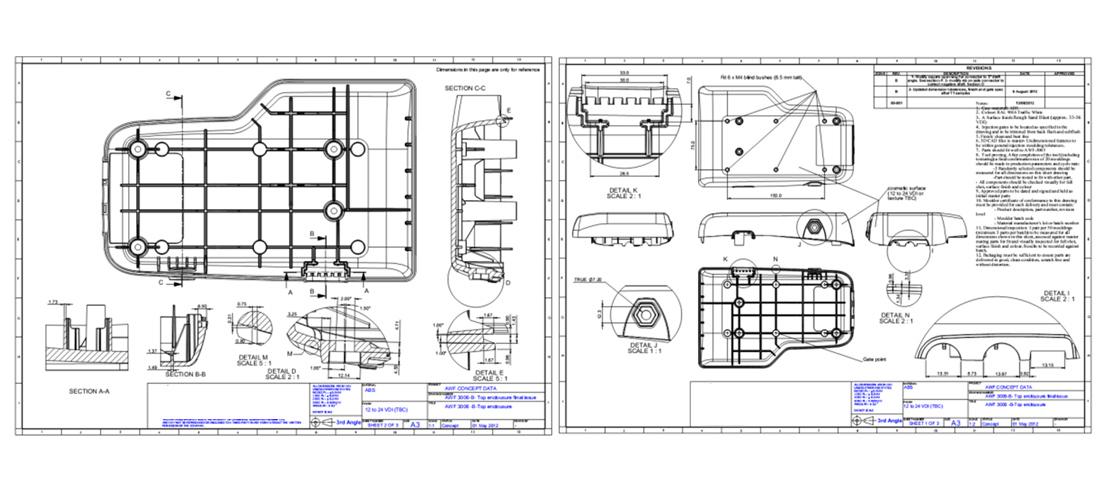
Through the design process, I never lose attention from the manufacturing aspects, but it is usually on the detailing stage when a considerable effort has to be put in place to ensure that the product and all its parts are fit for manufacturing.
Within this field I look in simple aspects like mouldability, (draft angles, mould flow, wall thickness, gate points, ejector points, etc.), but also more analytical areas such as tolerance analysis and process optimization.
Material selection and optimization
As soon as the requirements for a product are defined, I start looking for the most suitable material that will be fulfil the product requirements, be accessible and easy to manufacture and be cost effective. My experience in this field allows me to quickly identify a suitable material group to then refine the selection using different tools and advice from raw material distributors and manufacturers.
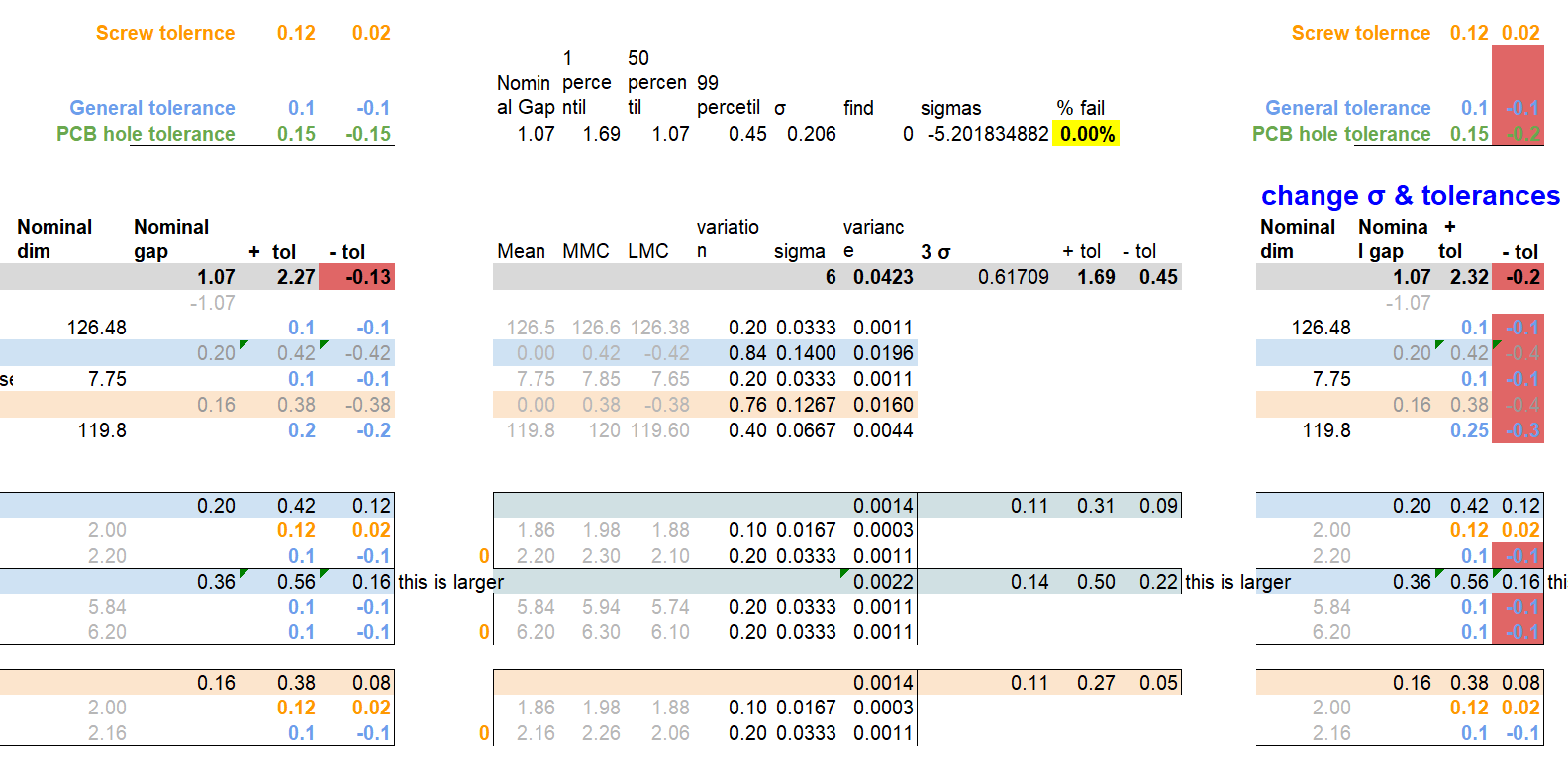
Tolerance analysis
In this area, simple stack analysis could be conducted to define the manufacturing tolerance windows for all critical features. The downside is that the tighter the tolerance, the more complex a manufacturing process requires to be, thus increasing the cost and timescales. Therefore in some cases statistical tolerance analysis is used, which allows increasing the tolerance windows by using statistical tools. In any case, the ideal solution is to start with a design that does not rely on a tight tolerances, however this is not always possible.
When tolerancing, I use both numerical and geometrical tolerances and work with the suppliers to ensure that tolerances can be met without undergoing extensive inspections and increase in cost and timescales.
Designing for injection moulding
Designing products to be made in high volumes is not a secret, but experience in this field helps in foreseeing challenges and features that may not be formed correctly. I follow a series of guidelines and reviews through the design process to ensure that the manufactured product will meet the requirements and stakeholder’s expectations.
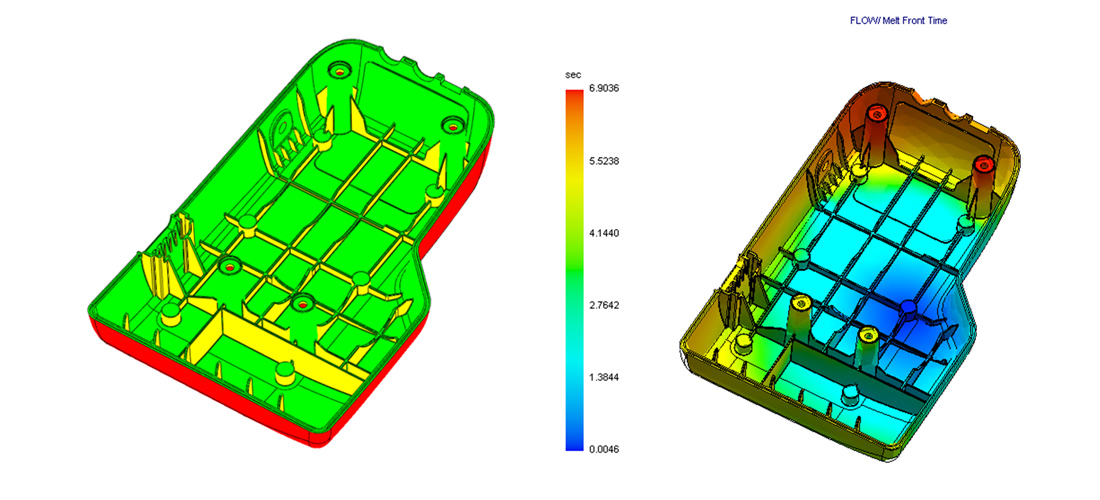
Within the detail design process I look into wall thickness, draft angles, mould flow analysis, sink marks analysis, deformation and bending analysis, gating and ejecting. All this tools, along with experience and moulders reviews allow me to create designs that will be made as intended.
Supplier selection
Part of the work that I conduct relies on finding the right supplier for my client. This could be from a raw material supplier to an OEM. Using the right partner could be the key to success and I help my clients on evaluating the suppliers and define which the best one for the project.
My network of supplier allows me to quickly identify potential partners for the most common processes and products. However in some instances we need to look for very special skills and a good match to ensure a successful outcome.
The network of manufactures that I work with include companies in Europe, Asia and America

Industrialization and New Product Introduction (NPI)
New Product Introduction comprises the whole development through to launch, but usually is referred to the latest phases where the product is industrialized and transferred to a manufacturer. NPI is usually described as the process below:
Define – Produce clear requirements and specifications.
Feasibility -Evaluate with stakeholders and the team if the targets, and requirements can be met.
Develop – Design and Engineer with constant reviews and evaluations. Including assembly process, process testing, jigs and fixtures where needed
Validate – Thorough and methodical product and process testing.
Implement – Prototype build or prototype production. Improve process and ramp up.
Evaluate – constant feedback to improve design, process and manufacturing
The important part of NPI activities is the transfer to manufacture. During this phase of work, we will hand over the design authority (and responsibility) to the manufacturer. We will start producing the first batches and confirm that all the quality procedures are in place that will guarantee that the product will be made according the agreed specifications.
This phase could require the greatest investments on a product’s lifecycle, and if not planned and executed properly could have catastrophic effects. The complexity of making parts to the required quality is often underestimated, costs could increase quickly and tensions between partners and suppliers could become difficult to manage. My experience in planning and transferring products to manufacture has taught me that it is here, that attention to all details and quick reaction to problems is paramount.
A key part of this process is to find the right manufacturing partners that could share the commercial vision that the project requires.
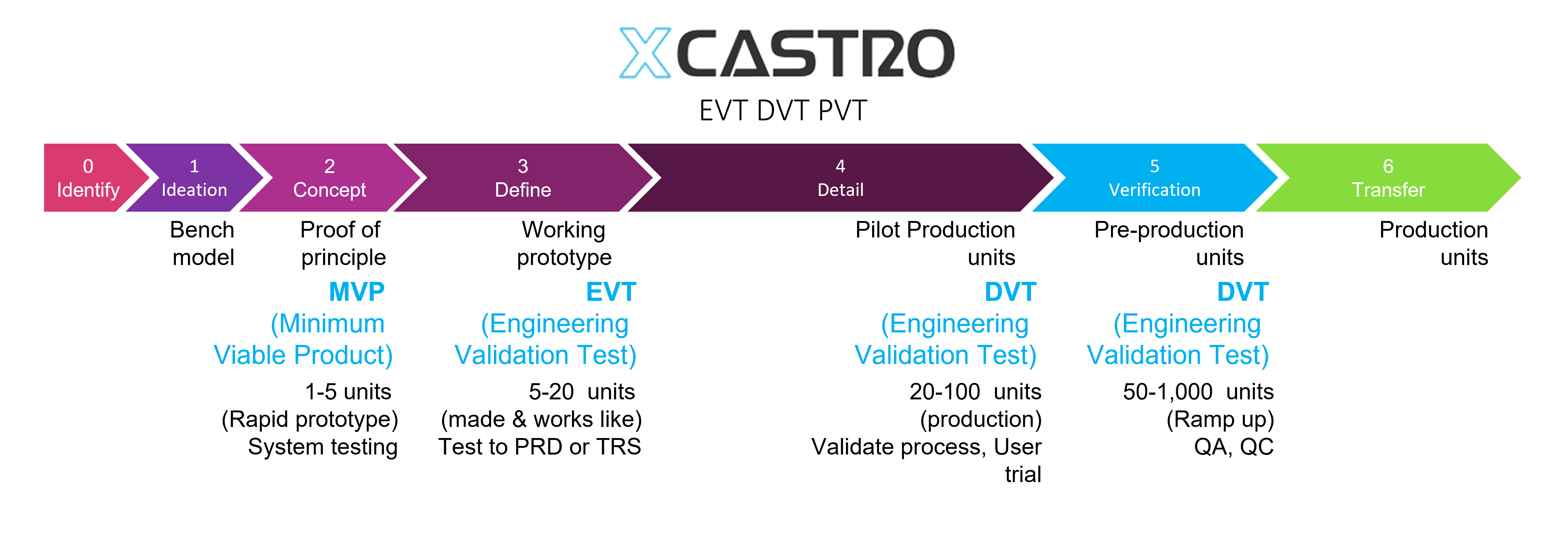
EVT DVT and PVT
Specially used for consumer electronics products, these terms relate to the resolutions of prototypes and production units that are required to validate that a design is fit for mass production and will meet the technical and user requirements (TRS, URS) or PRD (product requirement document).
The number of samples and type of tests depends on the type of product and risks. I recommend to conduct engineering testing and user trials as soon as possible to guarantee that the design is fit for purpose. From a manufacturing perspective is important to have all the documentation ready and a test plan in place to run the pilot production and ramp up as efficiently as possible.
Manufacturing validation process
In the medical sector and in other regulated industries it is vital to comply with high quality standards, sometimes referred as GMP (Good Manufacturing Practices). In my line of work, I don’t finish the process by handing over design specs to a manufacturer partner, but in many cases I have to work in close collaboration with them in validating the manufacturing process. The objective is to ensure that the product (or device) is made as it should and will always be made the same way. In this process we also hand over the “design authority” from the development team to manufacturing. So that no change is made to the design without having the manufacturing team fully involved.
To validate the manufacturing process we need to set up a series of test and evaluations plans where we will be defining what an acceptable part and device will be. The process looks at the equipment, the tooling and the process itself, and its describe by the FDA in the following way:
Process Validation
Means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications.
Installation Qualification (IQ)
Look that all the equipment is fit for purpose and has been set up correctly.
- Equipment design features
- Installation and Environmental Conditions
- Safety features
- Supplier documents, Calibration, preventative maintenance and spare parts.
Operational Qualification (OQ)
Challenge process parameters to assure the process will result in product that meets requirements.
- Determine process control limits
- Material specifications and handling
- Process change control and training
- Determine potential failure modes, action levels and worst case scenario
- Perform software V&V for intended use
Performance Qualification (PQ)
Demonstrate the process will consistently produce acceptable product under normal operating conditions.
- Approved procedures and limits from OQ
- Acceptable product
- Simulate actual manufacturing conditions
- Is the process repeatable and stable long term
Six Sigma y Lean Manufacturing
This is a process that I use from the design process to reduce and if possible, eliminate all the aspects that do not add value in order to make the product and process as efficient as possible.
I use a wider range of tools, like:
- Poka-Yoke
- Keizen
- Kanban
- Bottle neck analyis
- 5S
- TRIZ
- QFD
- FMEA
- Gauge R&R
- Root Cause Analysis
- KPIs
- 5 whys
PPAP-Production Part Approval Process
For consumer products we usually refer to PPAP practices to approve the manufactured products, in other words, validate the production process.
The methodologies are very similar to the one used to validate the production of medical products and relies on part measuring and testing and statistical data to determine the acceptance criteria for approval.
The key objective of PPAP and six sigma techniques is to ensure that the manufacturer can meet all times the design requirements (or design specification)
Collaboration
I work as a consultant or contractor with different clients and consultancies supporting the transfer to manufacture, manufacturing validation and new product introduction processes. I am based in Cambridge, UK.
Find out more on how I work or get in touch