Medical devices
Design and Development of medical products from concept to manufacture. Including documentation to CE and FDA requirements as well as Design Verification and Validation.
Medical sector is where I concentrate most of my work. The main difference in this sector is the required documentation that has to be produced to meet regulations. As part of this documentation, the key aspects are risk management and design verification.
My experience is mainly on CE and FDA regulations. I follow ISO 13485 and 21 CFR processes where I have developed class I, IIa, IIb and III devices with complete technical files including design verification and human factors validation.
I am familiar with the most relevant ISO standards, such as:
-13485 (quality)
-60601 (electrical safety)
-62304 (software)
-10993 (biocompatibility)
-14971 (risk management)
-62366 (usability)
Within the medical sector, I specialize in drug delivery, wound care and medical therapy, but have worked in diagnostics, surgical and other devices including connected devices and electromechanical systems.
Portfolio
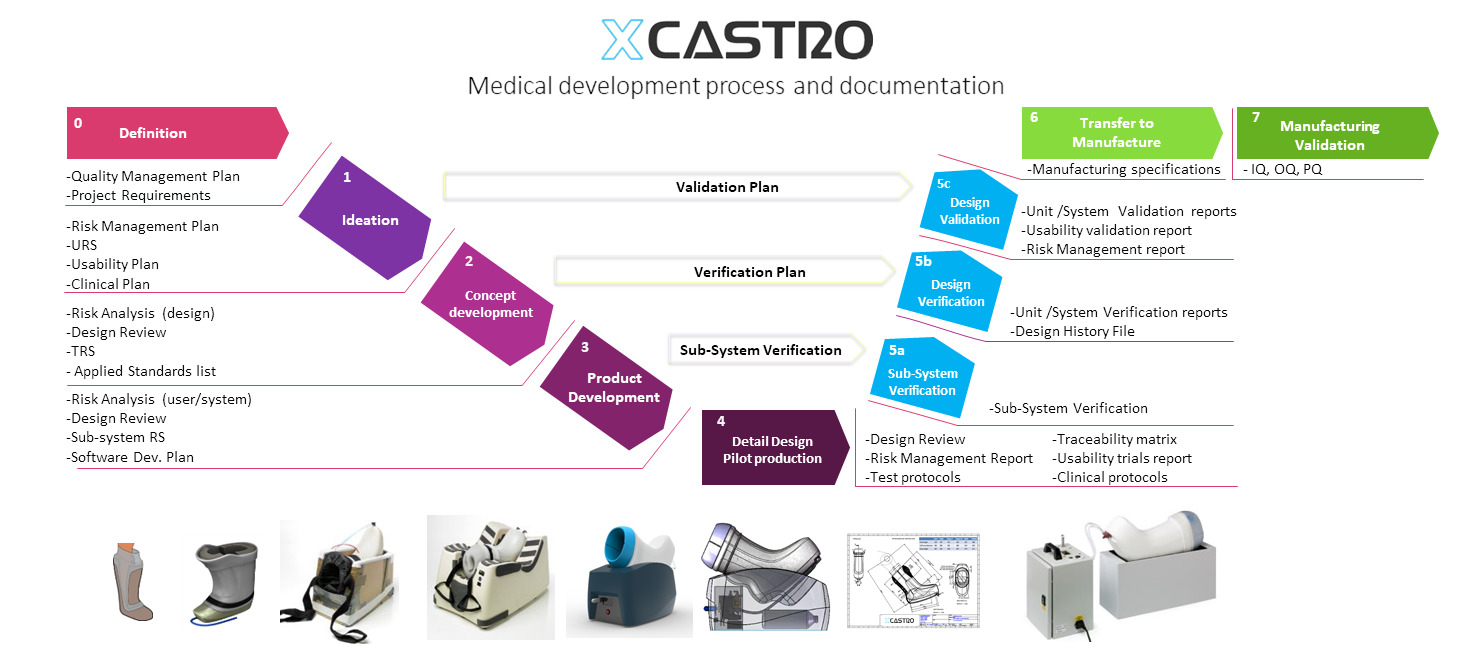
Medical Development process
When developing medical products, it is critical to follow a rigid documentation process to deliver a thorough technical file and technical documentation in compliance with different regulatory requirements.
My approach is to follow a “V” model that aligns with ISO 13485 and 21 CFR. This model sets out the documentation and quality requirements from early stages, including a detail look at risk management following 14971 processes and the required verification and validation stagey from the outset.
My experience not only concentrates on the design and engineering, but can also assist companies and clients on their regulatory documentation, technical file and design verification process.
Connected devices
Connectivity has become a key technology area that can be used to improve patient’s compliance and behaviour. I have worked on the design of several connected drug delivery devices, developing different platforms and technologies to track how a device has been used and assist patients on the learning process of how to use their device efficiently.
Connected devices require special attention to several areas. On one side the technical aspects such as using the right sensors and optimizing energy use to reduce battery size. Another one, is an efficient use of electronics and software to obtain a sleek performance and communication. Finally an intuitive and elegant interface that will engage patients.
.jpg)
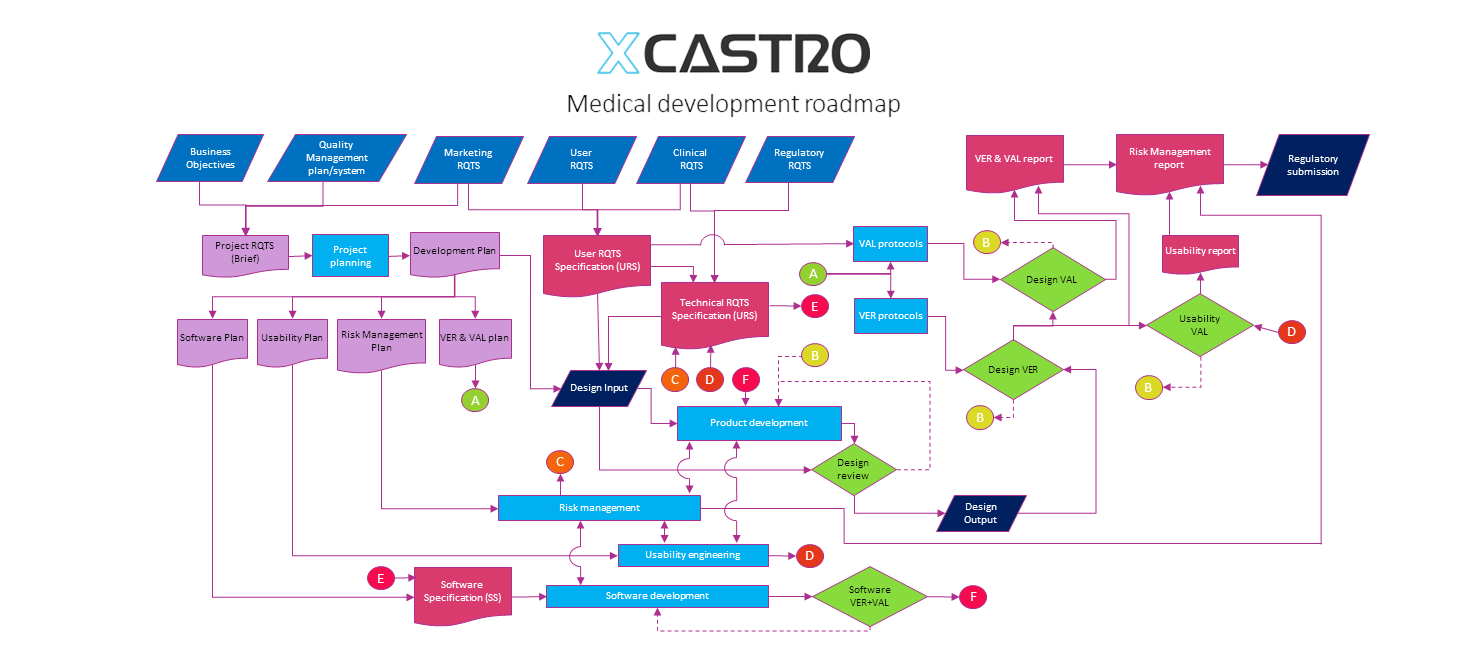
Regulatory roadmap and strategy
A critical aspect of medical devices development is to have a clear understating of the regulatory process, documentation requirements and define a regulatory strategy.
The chart below describes how complex the regulatory documentation workflow is and that having a good planning in this area will help delivering the required documentation at each stage.
To learn more about the regulatory strategy, look here
Design Verification
This is perhaps the most critical aspect of a medical device development programme as it will demonstrate that the product is fit for purpose and safe to use. Testing has to be conducted following a series of standards, with validated protocols using gage R&R or other methodologies and calibrated equipment. Planning in this area is essential and having a good verification strategy and plan will reduce costs and timescales considerably. My experience in this area covers from drug delivery devices to class III caseworks.
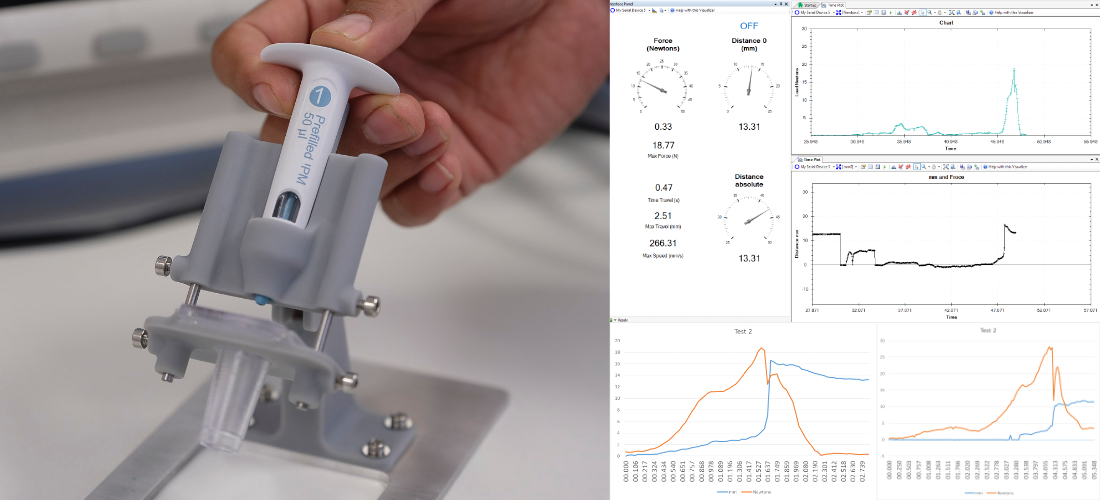
Human Factors
Regulations have become more demanding on demonstrating that devices are safe to use and free from use or user errors. This is a relatively new field where there is not a clear process to be followed and each device may require a different usability strategy. I have developed human factors reports that included formative and summative reports.
In my experience, the key on Human factors is the quality of the studies and not the quantity and conducting them at the right development phase, not to with every iteration. Also, allowing formative studies to be flexible and fast, while summative being rigorous and exhaustive.

Helping you
I support from start up to multinational pharmaceuticals on different aspects of the product development and leading design teams. I can collaborate on contract, consultancy or associate basis. I am based in Cambridge, UK
Find out more on how I work or contact me