Usability, Human Factors Engineering
Human Factors Engineer consultant and contractor, trained to meet FDA guidance and CE requirements to ISO 62366. Experienced in Usability Engineering in all aspects from documentation to formative and summative studies including creating user interfaces and instructions for use (IFU).
IFU and usability study for ICU device
.jpg)
Usability
A new medical device has to demonstrate that is safe to use as part of a regulatory approval. This is a relatively new field as it has only been enforced in the last few years. Methodologies and process are still been reviewed but in general there are two documents that need to be follow; the FDA guidance and ISO 62366.
My work in this field follows both approaches (FDA and ISO/CE). Depending on the regulatory requirements I will tailor a human factors strategy to ensure that the required studies are produced and the documentation is generated accordingly.
In this field the words count more that the numbers. When creating a Usability Report, what counts are the justifications and descriptions and not the quantity of documents or analytical reports. It is not about how many studies have been made or how many participants were on each study, but rather about the quality of each study in terms of observing that all safety aspects were evaluated properly.
Another important thing to remember is that it is not about patient or consumer satisfactions, as this should not be evaluated as part of a Human Factors study (unless it can be proven that it has an effect on the user safety, but I won’t recommend trying this). The main concern from a Usability standpoint is to be able to identify user errors, understand their risks and mitigate them accordingly. In order to do so, there is a series of analysis and studies that have to be conducted and method to evaluate the user interaction.
Each project requires a specific approach, but here is a list of areas where I have experience and that are important to conduct on each usability project.
Usability Plan
A good usability plan will present a strategy that will be followed by the team to identify and mitigate use errors. It is very useful to know what, who and where the usability activities will be conducted. So that no delays are caused in the development process and resources are allocated accordingly. It also serves to conduct a smooth and streamline usability process that will concentrate on key aspects.
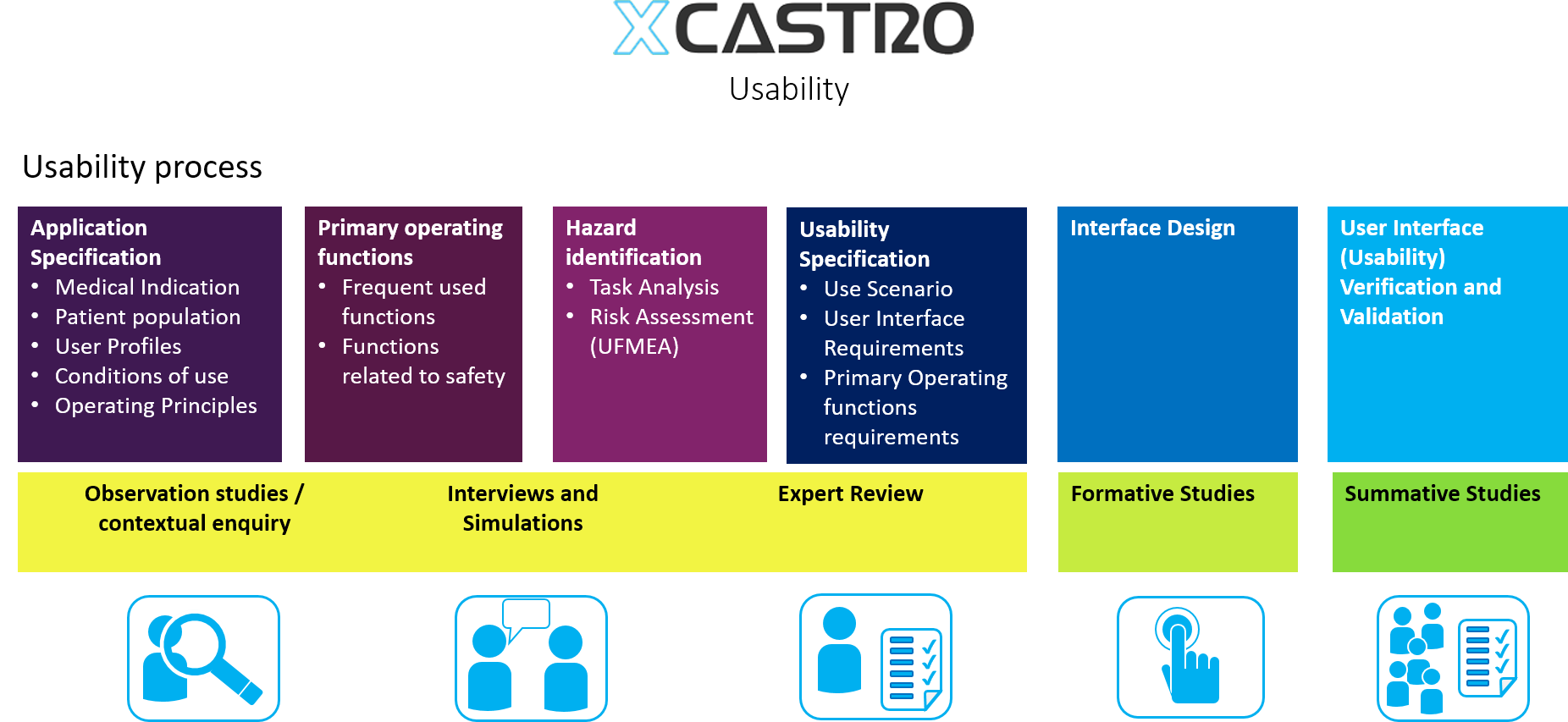
Task Analysis
This a methodology that identifies the steps that a user goes through when suing product. It concentrates on observing the sequence of events that need to be follow and identify any potential risks from conducting the task in a different way.
Task analysis
.jpg)
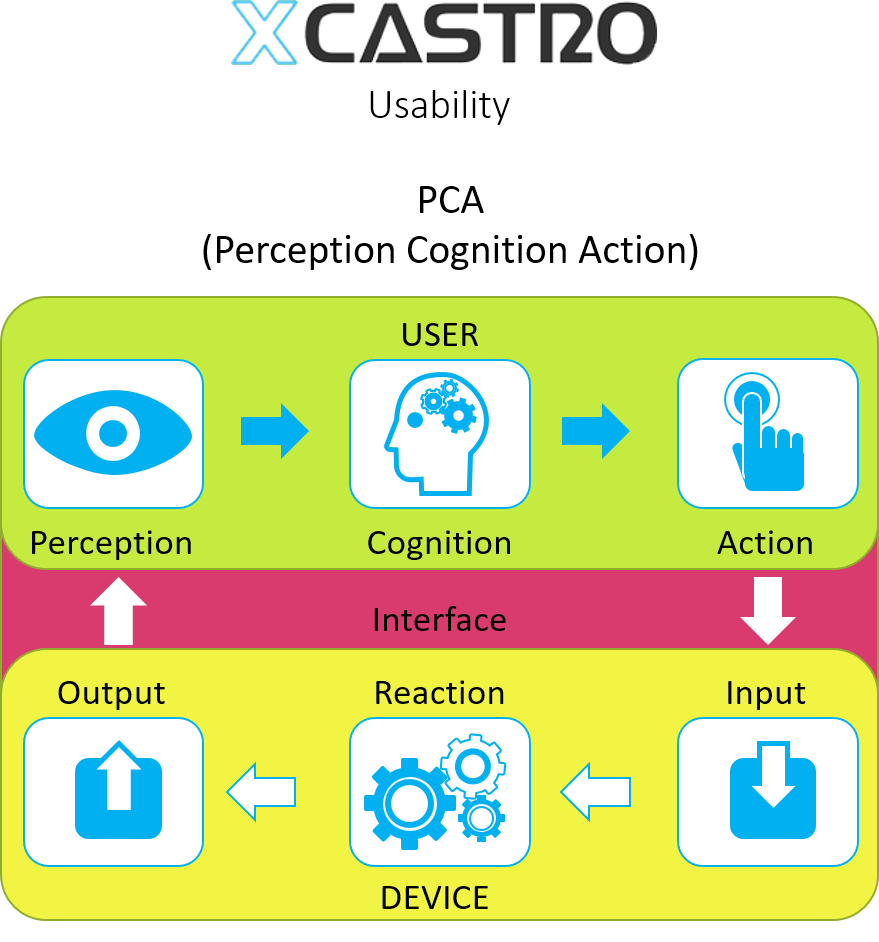
Perception Cognition Action
Similar to the task analysis, this process evaluates the process that a user follows, but in this case it looks at the interaction between the device and the user. This is a very useful tool when specifying a User Interface.
Formative Studies
Although this are not officially required by the regulatory bodies, they are expected to be documented. The key aspect of this studies is to identify potential use errors on different stages of the development process. They do not need to be rigorous but a good protocol and planning helps on making the most out of them.
User interface Specification
Once the user errors have been identified and mitigation actions defined, it is good practice to summarize this findings in a user interface specification. This document will also serve as guide to define the Validation and Summative studies approach.
Summative Studies
This is the final evaluation of the product as serves as part of the Usability Validation. It will prove that a device is safe to use and that all use errors have been dealt with. A good protocol, thorough planning and meticulous execution are key aspects of a summative study.
Foot ulcer device Formative Studies
.jpg)
.jpg)
Usability or Human Factors report
Finally, a report is the most important part of the process. It is here where is important to convey the reason for following a specific process and how the study’s results can be interpreted.
Supporting your development process
I work with different medical devices, including pharmaceuticals and drug delivery devices manufacturers on conducting human factors and usability studies and producing all the required documentation. I can assist companies on a contract, consultancy or project basis. I am based in Cambridge , UK


